Audit Management Guidelines

Audit management is a critical aspect of pharmaceutical manufacturing, playing a key role in maintaining product quality, regulatory compliance, and operational excellence. Effective audit management helps pharmaceutical companies identify gaps, improve processes, and ensure adherence to regulatory standards. This blog will explore the key guidelines governing audit management in the pharmaceutical industry and highlight their significance.
Key Audit Management Guidelines in the Pharmaceutical Industry
1. ICH Q10: Pharmaceutical Quality System
The International Council for Harmonisation (ICH) Q10 guideline provides a comprehensive model for an effective pharmaceutical quality system, including audit management as a critical element. Key aspects include:
- Internal Audits: Regularly conducting internal audits to assess compliance with established procedures and regulations.
- Management Responsibility: Ensuring top management is involved in audit processes and uses audit findings for continuous improvement.
- Corrective and Preventive Actions (CAPA): Implementing CAPA based on audit findings to address identified issues.
Useful links :
ICH Q10 Pharmaceutical Quality System
European Medicines Agency: ICH Q10
2. FDA 21 CFR Part 211
The U.S. Food and Drug Administration (FDA) regulations, specifically 21 CFR Part 211, outline the requirements for audit management in the manufacture of pharmaceutical products. These regulations emphasize:
- Quality Control Unit: The quality control unit is responsible for conducting audits and ensuring compliance with CGMP (Current Good Manufacturing Practice) regulations.
- Documentation: Maintaining thorough documentation of audit findings and actions taken to address them.
- CAPA: Implementing corrective and preventive actions based on audit results.
Useful links: FDA 21 CFR Part 211
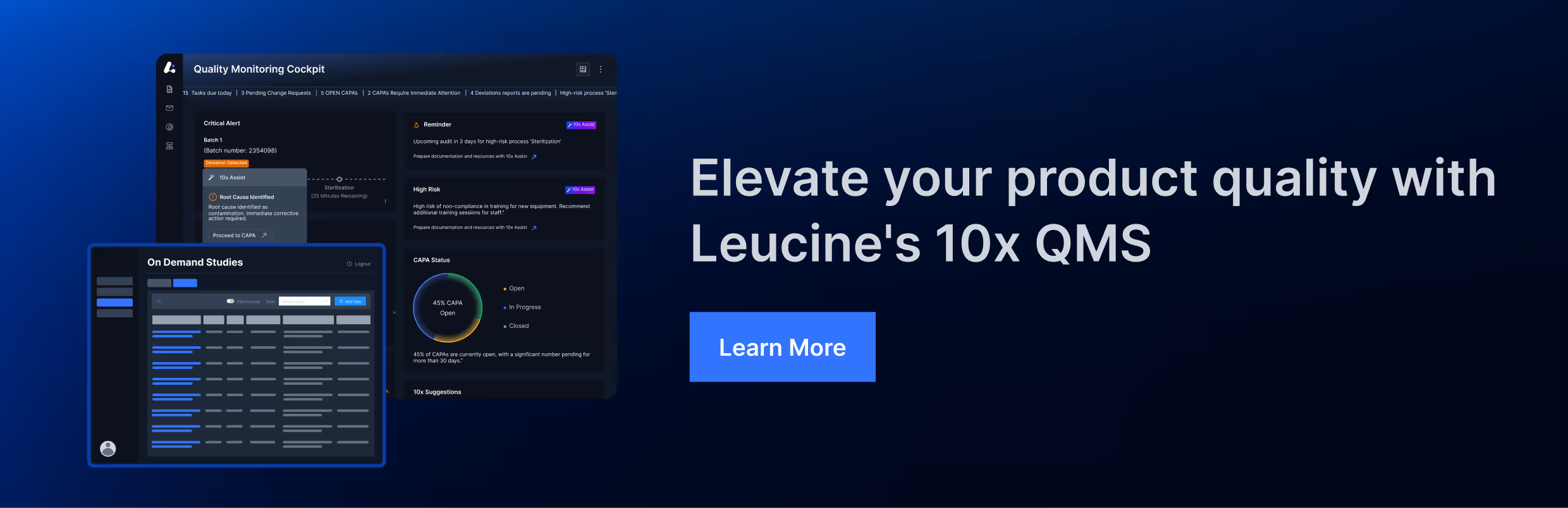
3. EU GMP Chapter 9
The European Union Good Manufacturing Practice (EU GMP) guidelines, specifically Chapter 9, provide detailed requirements for self-inspection and quality audits. Key aspects include:
- Self-Inspections: Conducting regular self-inspections to ensure compliance with GMP standards.
- Audit Schedule: Establishing a schedule for regular audits covering all aspects of GMP.
- Audit Reporting: Documenting audit findings and actions taken to address non-compliances.
Useful links: EU GMP
4. ISO 9001:2015
ISO 9001:2015 sets out the criteria for a quality management system and includes requirements for internal audits. While it is not specific to the pharmaceutical industry, its principles are universally applicable. Key aspects include:
- Audit Planning: Planning and scheduling regular internal audits.
- Audit Criteria: Defining the criteria and scope of audits.
- Audit Reporting: Documenting audit findings and communicating them to relevant stakeholders.
- Follow-Up Actions: Ensuring corrective actions are taken and their effectiveness is verified.
Useful links : ISO 9001:2015
5. WHO GMP
The World Health Organization (WHO) GMP guidelines emphasize the importance of audit management in maintaining product quality and safety. Key elements include:
- Internal Audits: Conducting regular internal audits to ensure compliance with GMP standards.
- Audit Documentation: Keeping detailed records of audit findings and corrective actions.
- Management Review: Ensuring audit findings are reviewed by management and used for continuous improvement.
Useful links: WHO GMP
6. PIC/S GMP
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) GMP guidelines provide a framework for audit management to ensure consistency and regulatory compliance. Key aspects include:
- Audit System: Implementing a system for managing audits.
- Audit Process: Conducting audits systematically and thoroughly.
- Audit Follow-Up: Ensuring that audit findings are addressed promptly and effectively.
The Importance of Adhering to Audit Management Guidelines
Adhering to audit management guidelines is essential for several reasons:
- Regulatory Compliance: Ensures compliance with local and international regulations, avoiding legal issues and penalties.
- Product Quality: Maintains product quality by identifying and addressing issues.
- Risk Management: Identifies and mitigates risks associated with product quality and safety.
- Operational Efficiency: Streamlines processes and reduces disruptions caused by non-compliances.
- Continuous Improvement: Drives continuous improvement of processes and systems, enhancing overall quality.
Best Practices for Effective Audit Management
To implement an effective audit management process, pharmaceutical companies should consider the following best practices:
- Establish Clear Procedures: Develop and maintain comprehensive procedures for conducting audits.
- Train Auditors: Ensure that auditors are well-trained and knowledgeable about relevant regulations and standards.
- Conduct Regular Audits: Schedule and conduct regular audits to assess compliance and identify areas for improvement.
- Document Findings: Maintain detailed records of audit findings, including non-compliances and areas for improvement.
- Implement CAPA: Develop and implement corrective and preventive action plans to address audit findings.
- Review and Monitor Audits: Regularly review audit processes and monitor the effectiveness of actions taken to address findings.
Recent Changes in Audit Management Guidelines
Staying updated with recent changes in audit management guidelines is crucial for maintaining compliance and ensuring best practices. Here are some recent updates:
1. Revised EU GMP Annex 1
The European Union has revised Annex 1 of its GMP guidelines, which focuses on the manufacture of sterile medicinal products. The revision includes enhanced requirements for audit processes, emphasizing contamination control and environmental monitoring.
2. FDA's Updated Guidance on Quality Metrics
The FDA has introduced draft guidance on quality metrics to support the evaluation of drug manufacturing quality. This guidance encourages the use of quality metrics to identify opportunities for improvement and to foster a culture of quality.
3. ICH Q9 (R1): Quality Risk Management
The International Council for Harmonisation (ICH) has updated its Q9 guideline on quality risk management. The revision aims to provide more clarity on the use of risk-based approaches in audit management processes.
4. WHO's Updated GMP Guidelines
The WHO has updated its GMP guidelines to incorporate more stringent requirements for audit management, focusing on risk assessment and documentation. These updates aim to enhance global health and safety standards.
Conclusion
Audit management is a critical component of pharmaceutical manufacturing, ensuring that all processes and systems comply with regulatory standards and operate efficiently. By adhering to established guidelines such as ICH Q10, FDA 21 CFR Part 211, EU GMP Chapter 9, ISO 9001:2015, WHO GMP, and PIC/S GMP, pharmaceutical companies can maintain product quality, regulatory compliance, and operational excellence. Implementing robust audit management processes and best practices is essential for achieving success in this highly regulated industry.
Explore all our recourses on QMS























.png)
