Electronic Batch Manufacturing - Audit Readiness Checklist



Transform Your Pharma Operations with AI-Powered Intelligence. Experience how Leucine's integrated platform brings together manufacturing, quality, and laboratory operations in one digital ecosystem. Schedule a personalized demo to see how our AI solutions can optimize your processes, ensure compliance, and drive operational excellence.
See How AI Transforms Your Shop Floor Operations.
Experience firsthand how Leucine's 10x MES digitalizes batch records, streamlines production, and provides real-time monitoring of your manufacturing operations. Schedule a personalized demo to discover how our AI-powered platform can enhance your productivity while maintaining compliance.
Experience AI-Driven Quality Management in Action.
See how Leucine's 10x QMS automates compliance workflows, streamlines change control, and ensures regulatory adherence. Schedule a demo to learn how our AI-powered platform can help you manage quality processes more efficiently while reducing compliance risks.
Discover Smart Laboratory Operations Management.
Watch how Leucine's 10x LES orchestrates your lab operations, automates documentation, and ensures data integrity. Schedule a demo to see how our AI-powered platform can accelerate your testing processes while maintaining audit-readiness.





Ensure all records are complete, accurate, and easily accessible.
Evaluate the efficiency and effectiveness of your processes and systems.
Verify adherence to all relevant regulations and standards.
Assess the condition of your facilities and the competence of your staff.
Check the effectiveness of internal and external communication channels.
Ensure ongoing tracking and resolution of CAPAs to maintain compliance.

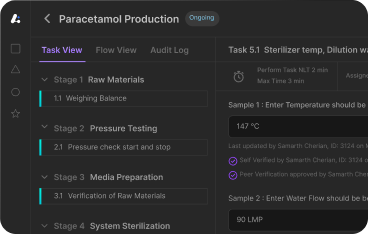
Ensure batch records are complete, traceable, and ALCOA+ compliant. Maintain documentation accuracy with real-time logs and version control.
Eliminate manual errors with automated workflows. Reduce delays by enforcing SOPs and enabling real-time tracking of batch execution status.
Achieve consistency with standardized batch formats, interlocks, and procedural controls that minimize variability and improve yield reliability.
Identify deviations early with AI-powered analytics. Conduct root cause investigations promptly and implement CAPA for continuous improvement.
Stay ahead of evolving expectations from FDA, EMA, WHO, and MHRA. Build systems aligned with Annex 15, ICH Q7, and 21 CFR Part 11.
Use tools like FDA Tracker and Leucine MES to uncover bottlenecks, analyze trends, and drive predictive quality across all production stages.