Standardize Shop Floor Workflows.
Ensure Data integrity & Data security with Elogbook Software
Ensure every batch is delivered On Time, In Full (OTIF) with AI-driven intelligence. Achieve flawless execution and real-time visibility.





















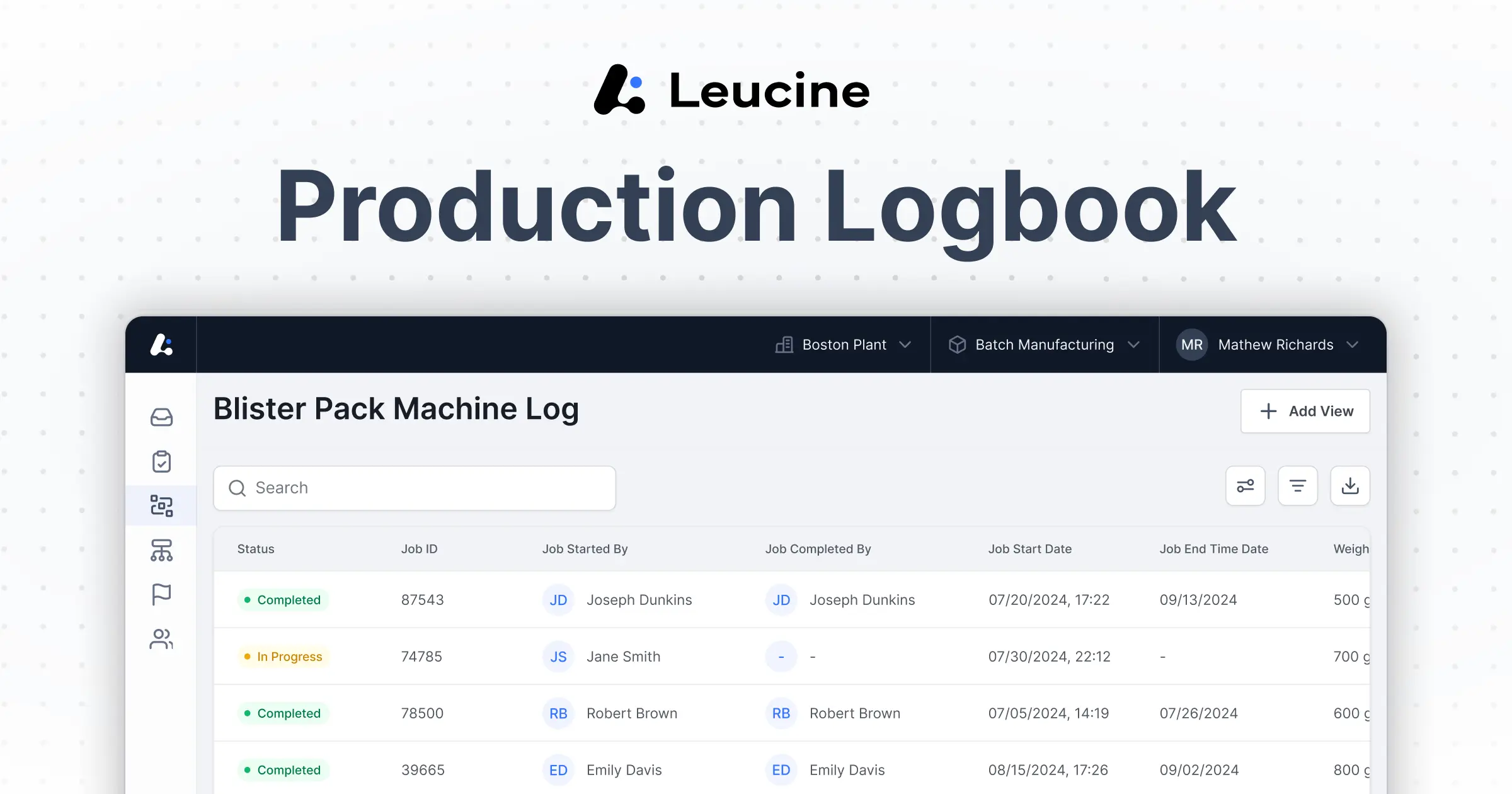
MES Integrated Electronic Logbook software for a Single Source of Truth for Manufacturing Data
Digitize and integrate your E log book with MES to ensure real-time data capture, compliance, and seamless workflow connectivity. By centralizing logbook management and reducing reliance on paper-based logbooks, you’ll streamline documentation, and enhance overall operational efficiency.
Reduce Documentation Errors
Eliminate manual logbook entries and ensure data accuracy with automated digital records. Reduce uncontrolled data entry by 80%, minimizing compliance risks. Audits trail are essential for ensuring regulatory compliance and maintaining data integrity.
Prevent Production Bottlenecks
Gain real-time visibility into shop floor operations and identify potential vulnerabilities. before they impact production. Proactively resolve issues to reduce recurring failures by 30%. Compliance issues can be detected through anomaly detection, spotting data outliers that may signify errors or potential compliance-related failures.
Reduce Operator Effort
Capture timestamps, integrate with systems like SAP, & reduce manual entry using AI-enabled electronic logbook software. Data security is crucial for protecting sensitive information, with robust features like encryption and access controls.
Accelerate Audits and Compliance
Easily maintain a single source of truth for all batch processed records. Simplify compliance logs through centralized record-keeping, helping you stay audit ready and compliant with industry guidelines at all times.

Connect digital logbook with batch execution, quality, and maintenance systems.
Digitize Logs using AI.


Standardize shop floor workflows.
Prevent missing steps. Enforce SOP compliance with Process Interlocks.


Capture Data Efficiently with Data accuracy & enhance operational efficiency
Always audit-ready, Reduce inspection risks.

Frequently Asked Questions
Can I trial Webflow before paying?

Sure! You can test out Webflow on our free plan where you can experiment with 2 projects. Your unhosted projects will have a two-page limit, but you can purchase a site plan on a per-project basis to unlock up to 100 static pages and additional CMS pages.
What is a project?
A project is a website that you build in Webflow. You can publish projects to a webflow.io staging subdomain for free, export the code on a paid plan, or add a site plan to connect your custom domain and unlock hosting features.
What can I white label?
Pro accounts can add their own logo to Client Billing forms and the Editor. Pro accounts can also remove references to Webflow in the source code and form submission emails, and hide the Webflow badge from their staging sites.
Frequently Asked Questions
1. What is Leucine Production Logbook, and how does it differ from traditional E log book software?
Leucine Production Logbook is an AI-powered eLogbook for pharma manufacturing, offering automated data capture, real-time visibility, and seamless MES, ERP, and QMS integration—reducing manual work, errors, and compliance effort.
2. How does Leucine Production Logbook ensure security & integrity of data?
Leucine enforces ALCOA principles with digital signatures, timestamps, and secure authentication, while protecting data through encryption and access control—ensuring compliance with 21 CFR Part 11, GMP, and global regulations.
3. Can Leucine Production Logbook integrate with our existing MES, ERP, or QMS systems?
Yes, Leucine Production Logbooks offers open APIs and configurable connectors to integrate with MES, QMS, and ERP systems—ensuring seamless data flow, eliminating duplicates, and enabling a single source of truth.
4. How do real-time visibility and AI-driven insights in Leucine Production Logbook help us deliver On Time, In Full (OTIF)?
Leucine monitors workflows and analyzes data in real-time to spot bottlenecks early, recommend actions, and help meet OTIF targets—ensuring smoother operations and better product quality.
5. How do Leucine Production Logbook support audit and compliance?
All log entries, edits, and approvals are auto-timestamped, creating a clear audit trail that simplifies inspections, and keeps you audit ready for FDA, GMP, and global standards.
6. How does Leucine Production Logbook reduce manual entry and eliminate paper-based logbook errors?
Leucine automates data capture with barcode/QR scanning, AI validation, and system integrations—eliminating paper processes, reducing errors.
7. Does Leucine Production Logbook support mobile access or remote monitoring?
Yes. Operators and supervisors can securely access real-time data, approve workflows, and resolve issues on tablets or smartphones—from anywhere.
8. How quickly can we implement Leucine Production Logbook, and what training is required?
Implementation timelines vary depending on your existing systems and workflows, but Leucine’s user-friendly interface and step-by-step deployment process help teams get started quickly. Training typically involves guided sessions on platform navigation, data capture, and integration setup, ensuring smooth adoption across departments.
9. How do electronic logbook from Leucine enhance equipment status tracking?
Leucine centralizes equipment usage, maintenance, and calibration records within a single digital platform. Real-time status updates and automated notifications allow you to schedule maintenance more effectively, reducing downtime and ensuring equipment is always available for production or quality control tasks.
10. What is the return on investment (ROI) of implementing Leucine Production Logbook?
Many organizations see immediate benefits in operational efficiency, fewer errors, and faster batch execution. Over time, these improvements translate into cost savings, higher OTIF rates, and enhanced overall productivity—delivering a strong ROI for pharmaceutical manufacturers.
View and learn more about MES with our comprehensive list of resources


















.png)
.png)
.png)


.png)
.png)
.png)