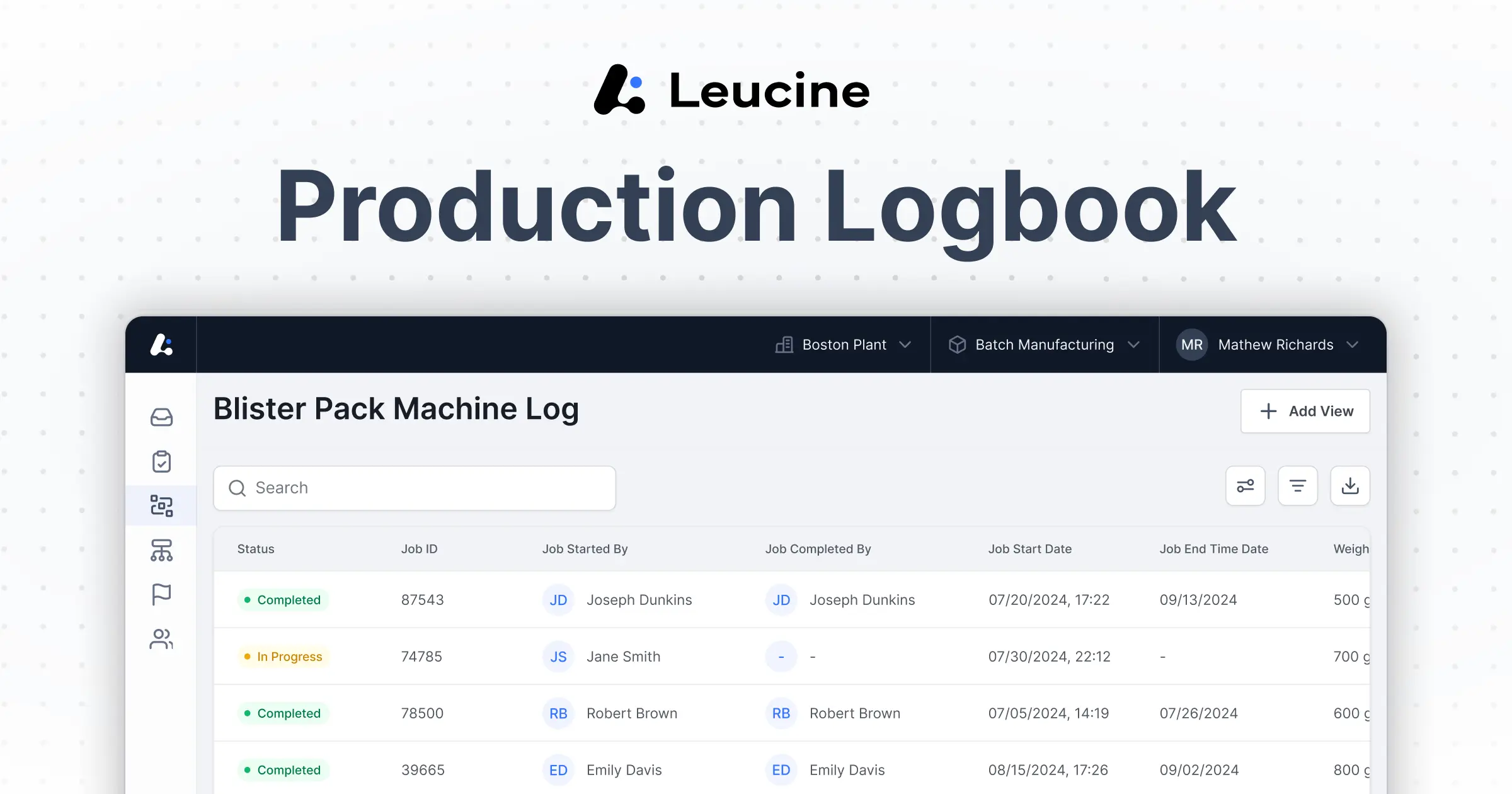
AI-Powered Electronic
Batch Record Software
Optimize every step of batch manufacturing—from weighing to release—while ensuring compliance, minimizing deviations, and accelerating production.





















Electronic batch manufacturing software that eliminates bottlenecks before they happen
Minimize Batch Start Delays
Ensure all production inputs—materials, equipment, and personnel—are verified and ready with automated production readiness checks, reducing unplanned stoppages.
Reduce Process Deviations
Enforce process interlocks, real-time alerts, and guided workflows to eliminate execution errors and ensure first-time-right batch manufacturing.
Expedite Batch Release
Capture data natively, track exceptions as they occur, and streamline QA with batch record review software—shrinking review cycles and speeding compliance..
Ensure accurate material dispensing with ERP & Balance Integration


Monitor Equipment and Area Readiness to start batches without delay
Standardize Batch Execution. Reduce Deviations & GDP Errors.


Track WIP inventory across the shop floor in real-time
Auto capture data via L2 Integrations and Computer Vision


Monitor your Facility and ensure compliance
Always audit-ready with digital records

Frequently Asked Questions
How does Leucine's Electronic Batch Record Software improve compliance and data integrity?
Leucine's Electronic Batch Record Software ensures FDA 21 CFR Part 11 and EU Annex 11 compliance by replacing paper systems with secure digital workflows. Our software incorporates electronic signatures, tamper-proof audit trails, and automated GMP enforcement. This improves data integrity, enhances product quality, and reduces compliance costs by up to 40%.
How does Leucine's Electronic Batch Record Software help with batch traceability?
Our software digitally documents every manufacturing step from raw materials to finished products. The software automatically links master batch records, equipment data, and personnel actions for complete traceability. This enables regulatory bodies to easily audit manufacturing processes with confidence.
How does Leucine's Electronic Batch Record Software help reduce batch release times?
Our software accelerates batch releases through automated digital workflows and provides real-time quality data capture, automated compliance checks, and streamlined electronic signatures. This reduces release times by 30-50% while maintaining full GMP compliance for pharmaceutical manufacturers.
Is Leucine's Electronic Batch Record Software compatible with other ERP and MES systems?
Yes, our platform features modular architecture designed for seamless integration with existing ERP, MES, QMS, and LIMS systems,eliminating data silos and streamlining manufacturing processes.
Does Leucine's Electronic Batch Record Software cover Electronic Batch Manufacturing, Batch Production Record, and Batch Record Review too?
Yes, our comprehensive platform includes all these capabilities. Our software handles complete production workflows. Rather than requiring separate systems, Leucine's unified electronic batch record software delivers all these functions in one integrated solution for pharmaceutical manufacturers.
View and learn more about Batch Manufacturing Execution with our comprehensive list of resources
Experience AI-Powered Electronic Batch Records with Leucine
Join leading pharmaceutical companies in transforming batch execution. Ensure regulatory compliance, reduce deviations and accelerate batch release—all with one intelligent solution.














.png)
.png)
.png)


.png)
.png)
.png)