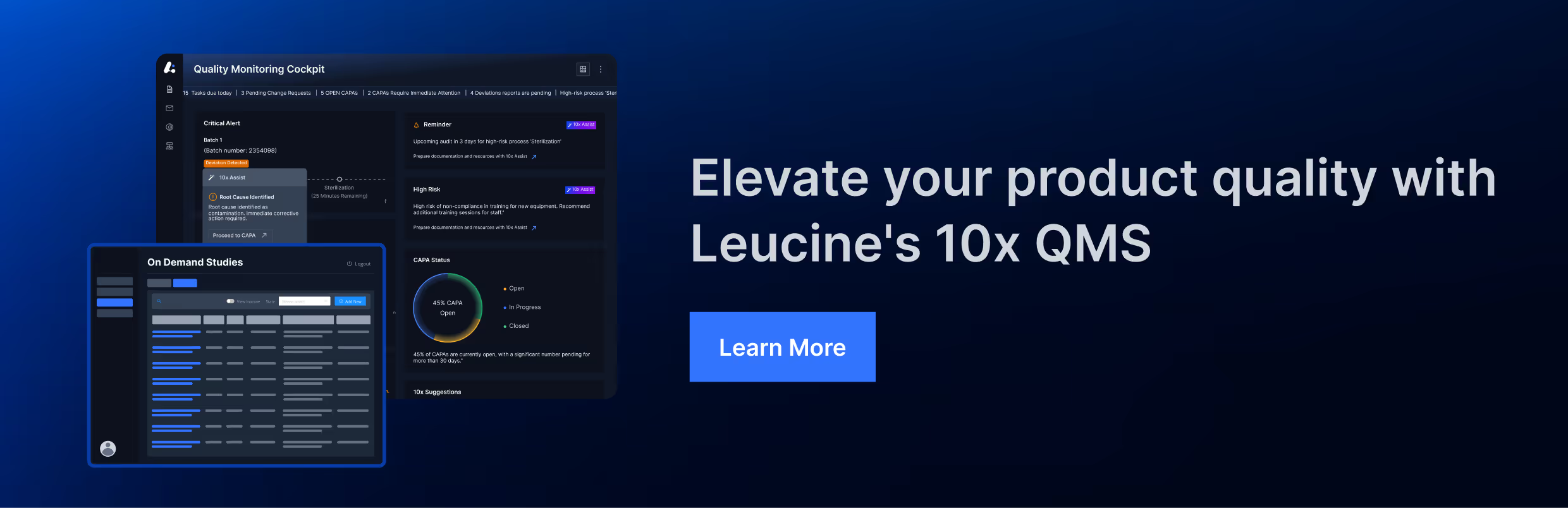
Change Control Guidelines
Change control is a pivotal aspect of pharmaceutical manufacturing, ensuring that all changes to processes, equipment, and systems are systematically evaluated and managed to maintain product quality and regulatory compliance. Implementing robust change control guidelines helps pharmaceutical companies mitigate risks, maintain consistency, and achieve operational excellence. This blog will explore the key guidelines governing change control in the pharmaceutical industry and highlight their significance.
Key Change Control Guidelines in the Pharmaceutical Industry
1. ICH Q10: Pharmaceutical Quality System
The International Council for Harmonisation (ICH) Q10 guideline provides a comprehensive model for an effective pharmaceutical quality system, which includes change management as a critical element. Key aspects include:
- Change Evaluation: Assessing the impact of proposed changes on product quality.
- Approval Process: Ensuring changes are reviewed and approved by authorized personnel.
- Documentation: Maintaining thorough documentation of all changes and their justifications.
- Implementation: Ensuring changes are implemented in a controlled manner.
Useful links : ICH Q10
2. FDA 21 CFR Part 211
The U.S. Food and Drug Administration (FDA) regulations, specifically 21 CFR Part 211, outline the requirements for change control in the manufacture of pharmaceutical products. These regulations emphasize:
- Written Procedures: Establishing and following written change control procedures.
- Change Review: Reviewing changes by the quality control unit to determine their impact.
- Change Approval: Ensuring changes are approved by authorized personnel before implementation.
3. EU GMP Annex 15
The European Union Good Manufacturing Practice (EU GMP) guidelines, specifically Annex 15, provide detailed requirements for change control. Key aspects include:
- Risk Assessment: Evaluating the risks associated with changes.
- Quality Impact: Assessing the potential impact on product quality.
- Change Approval: Obtaining approval from the quality assurance department before making changes.
- Post-Implementation Review: Reviewing the effectiveness of changes after implementation.
Useful links: EU GMP Annex 15
4. WHO GMP
The World Health Organization (WHO) GMP guidelines also emphasize the importance of change control in maintaining product quality and safety. Key elements include:
- Change Control Procedures: Establishing procedures for change control.
- Impact Assessment: Evaluating the potential impact of changes on product quality.
- Documentation: Keeping detailed records of changes and their impact.
Useful links: WHO GMP
5. PIC/S GMP
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) GMP guidelines provide a framework for change control to ensure consistency and regulatory compliance. Key aspects include:
- Change Management System: Implementing a system to manage changes.
- Impact Analysis: Conducting impact analyses for proposed changes.
- Approval and Documentation: Ensuring changes are approved and documented properly.
The Importance of Adhering to Change Control Guidelines
Adhering to change control guidelines is essential for several reasons:
- Regulatory Compliance: Ensures compliance with local and international regulations, avoiding legal issues and penalties.
- Product Quality: Maintains product quality and consistency by controlling changes.
- Risk Management: Identifies and mitigates risks associated with changes.
- Operational Efficiency: Streamlines processes and reduces disruptions caused by changes.
- Documentation and Traceability: Provides clear documentation and traceability of changes for audits and inspections.

Best Practices for Effective Change Control
To implement an effective change control process, pharmaceutical companies should consider the following best practices:
- Establish Clear Procedures: Develop and maintain comprehensive change control procedures.
- Involve Key Stakeholders: Ensure that all relevant stakeholders are involved in the change control process.
- Conduct Thorough Impact Assessments: Evaluate the potential impact of changes on product quality and regulatory compliance.
- Maintain Detailed Documentation: Keep detailed records of all changes, including their justification and impact.
- Review and Approve Changes: Ensure that changes are reviewed and approved by authorized personnel.
- Monitor and Review Changes Post-Implementation: Monitor the impact of changes after implementation to ensure they are effective and do not negatively affect product quality.
Recent Changes in Change Control Guidelines
Staying updated with recent changes in change control guidelines is crucial for maintaining compliance and ensuring best practices. Here are some recent updates:
1. Revised ICH Q12
The ICH Q12 guideline, which complements ICH Q10, focuses on technical and regulatory considerations for pharmaceutical product lifecycle management. It provides a framework to facilitate the management of post-approval changes, aiming to reduce the burden of regulatory submissions while ensuring product quality.
2. FDA's Updated Guidance on Post-Approval Changes
The FDA has updated its guidance on managing post-approval changes to streamline the change control process. The guidance outlines procedures for reporting changes and emphasizes the importance of maintaining product quality throughout the product lifecycle.
3. EU GMP Annex 16 Revision
The European Union has revised Annex 16 of its GMP guidelines to include more detailed requirements for the certification and batch release process. The revision emphasizes the importance of robust change control procedures to ensure compliance with the updated guidelines.
4. WHO's Updated GMP Guidelines
The WHO has updated its GMP guidelines to incorporate more stringent requirements for change control, focusing on the importance of risk assessment and documentation. These updates aim to enhance global health and safety standards.
Conclusion
Change control is a critical component of pharmaceutical manufacturing, ensuring that changes are managed in a controlled and systematic manner. By adhering to established guidelines such as ICH Q10, FDA 21 CFR Part 211, EU GMP Annex 15, WHO GMP, and PIC/S GMP, pharmaceutical companies can maintain product quality, regulatory compliance, and operational efficiency. Implementing robust change control processes and best practices is essential for achieving excellence in this highly regulated industry.
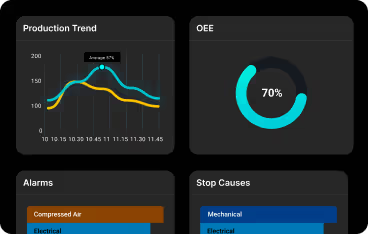
Observations in 2025

.png)
.png)
.png)






















