QMS Guidelines
In the pharmaceutical industry, Quality Management Systems (QMS) are the backbone of ensuring product quality, regulatory compliance, and patient safety. Implementing an effective QMS is not just a regulatory requirement but a crucial element in achieving operational excellence and maintaining a competitive edge.
This blog will delve into the key guidelines that govern QMS in the pharmaceutical industry, highlighting their significance and application.
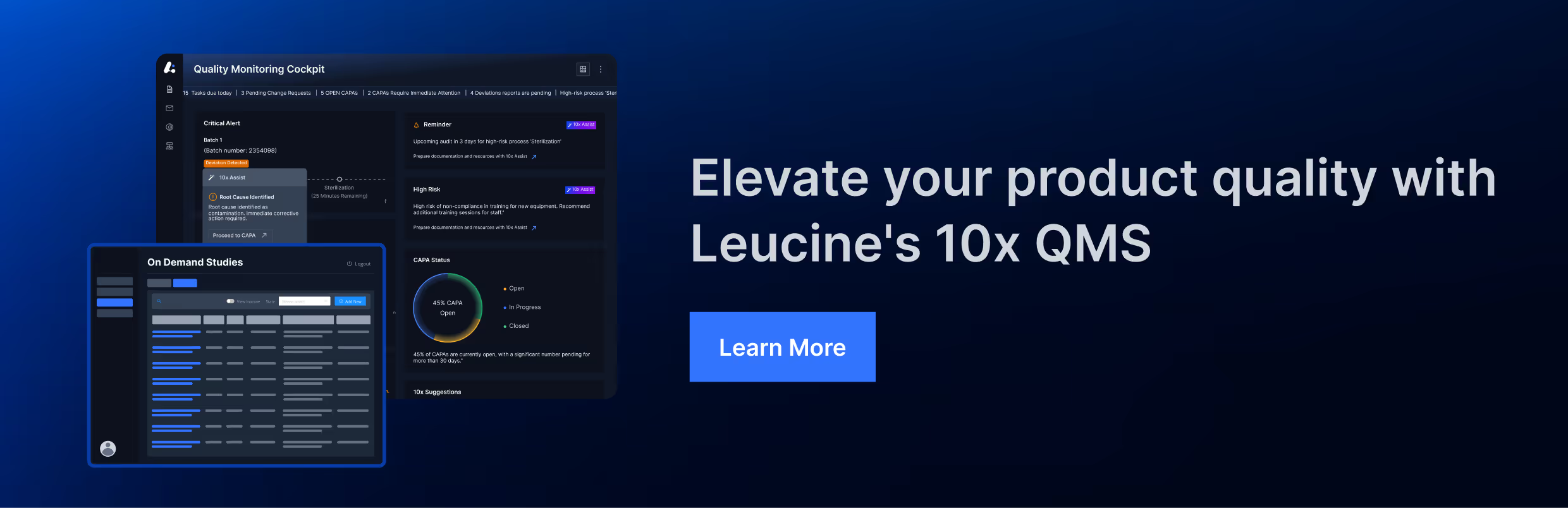
Key QMS Guidelines in the Pharmaceutical Industry
1. ICH Q10: Pharmaceutical Quality System
The International Council for Harmonisation (ICH) Q10 guideline provides a comprehensive model for an effective pharmaceutical quality system. It aims to harmonize the regulation of pharmaceutical quality systems across different regions. The guideline emphasizes the following aspects:
- Management Responsibility: Leadership commitment to quality.
- Continual Improvement: Ongoing enhancement of product quality and processes.
- Corrective and Preventive Action (CAPA): Addressing root causes of quality issues.
- Change Management: Controlling changes to processes and systems to maintain quality.
Useful links :
ICH Q10 Pharmaceutical Quality System
European Medicines Agency: ICH Q10
2. ISO 9001:2015
ISO 9001:2015 sets out the criteria for a quality management system and is the only standard in the ISO 9000 series that can be certified to. It is based on several quality management principles, including a strong customer focus, the involvement of top management, a process approach, and continual improvement. While it is not specific to the pharmaceutical industry, its principles are universally applicable.
Useful links : ISO 9001:2015

3. ISO 13485:2016
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. It is particularly relevant for pharmaceutical companies involved in the manufacture of medical devices.
Useful links: ISO 13485:2016
4. Good Manufacturing Practices (GMP)
GMP guidelines are a set of regulations that ensure products are consistently produced and controlled according to quality standards. Key aspects include:
- EU GMP: Detailed guidelines for quality management, personnel, premises, equipment, documentation, production, quality control, contract manufacturing, and distribution.
- FDA CFR Title 21 Parts 210 and 211: Regulations for manufacturing, processing, packing, or holding of drugs, and specific requirements for finished pharmaceuticals.
Useful links : EU GMP Guidelines
5. WHO GMP
The World Health Organization (WHO) GMP guidelines ensure that pharmaceutical products are consistently produced and controlled to quality standards. They cover all aspects of production from the starting materials, premises, and equipment to the training and personal hygiene of staff.
Useful links: WHO GMP
6. PIC/S GMP
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) aims to harmonize inspection procedures globally by developing common standards in the field of GMP. It provides a detailed framework for the manufacturing and quality control of medicinal products.
Recent Changes in QMS Guidelines
Staying updated with the latest changes in QMS guidelines is crucial for maintaining compliance and ensuring best practices. Recent updates include:
1. ICH Q12
Introduced to complement ICH Q10, the ICH Q12 guideline focuses on the technical and regulatory considerations for pharmaceutical product lifecycle management. It provides a framework to facilitate the management of post-approval changes, reducing the burden of regulatory submissions while ensuring product quality.
2. FDA's Draft Guidance on Quality Metrics
The FDA has introduced draft guidance on quality metrics to support the evaluation of drug manufacturing quality. This guidance encourages the use of quality metrics to identify opportunities for improvement and to foster a culture of quality.
3. EU GMP Annex 1 Revision
The European Union has recently revised Annex 1 of its GMP guidelines, focusing on the manufacture of sterile medicinal products. The revision includes enhanced requirements for contamination control strategies and environmental monitoring.
4. ISO 9001:2015 Transition Period
Organizations transitioning from ISO 9001:2008 to ISO 9001:2015 must ensure that their quality management systems align with the updated standards, focusing on risk-based thinking and a stronger customer focus.

The Importance of Adhering to QMS Guidelines
Adhering to QMS guidelines is essential for several reasons:
- Regulatory Compliance: Ensures compliance with local and international regulations, avoiding legal issues and penalties.
- Product Quality: Guarantees that products meet predefined quality standards, ensuring safety and efficacy.
- Operational Efficiency: Streamlines processes, reduces waste, and improves efficiency.
- Customer Satisfaction: Consistently high-quality products enhance customer trust and satisfaction.
- Risk Management: Identifies and mitigates risks associated with product quality and safety.
Conclusion
Implementing and maintaining a robust Quality Management System is crucial for pharmaceutical companies. By adhering to established guidelines such as ICH Q10, ISO 9001:2015, ISO 13485:2016, GMP, WHO GMP, and PIC/S GMP, pharmaceutical manufacturers can ensure product quality, regulatory compliance, and operational excellence. Continual improvement and commitment to quality are fundamental to achieving and sustaining success in this highly regulated industry.

Dive Deep into the Following Critical Aspects

Ensure all records are complete, accurate, and easily accessible.

Evaluate the efficiency and effectiveness of your processes and systems.

Verify adherence to all relevant regulations and standards.

Assess the condition of your facilities and the competence of your staff.

Check the effectiveness of internal and external communication channels.

Ensure ongoing tracking and resolution of CAPAs to maintain compliance.
.png)



















.avif)

