FDA Observations Change Control
1. Unwritten and Unfollowed QCU Procedures
Root Cause:
No written Quality Control procedures, inadequate shipping studies, inefficient change control management, and TrackWise limitations for change control extensions.
Corrective Actions:
Develop comprehensive Quality Control procedures, perform required shipping studies, amend change management to meet deadlines, and upgrade TrackWise or implement manual tracking for change control extensions.
Preventive Actions:
Train the Quality Control Unit on new procedures, regularly review shipping studies, implement a tracking system for change controls, and update TrackWise or equivalent software for efficient change management.
Reference FDA 483:
Company Name: Sun Pharmaceutical Industries Limited
Issue Date: 15-Dec-23
Inspection Dates: 04 Dec 2023 to 15 Dec 2023
Investigators: Pratik S Upadhyay
2. Inadequate Change Control for Written Procedures
Root Cause:
Unclear change control procedures, inadequate training on documentation, insufficient quality unit oversight, and departmental disconnect on procedure changes and impact assessment.
Corrective Actions:
Review and update change control procedures for clarity, conduct comprehensive training on documentation, implement quality oversight for compliance, and facilitate cross-departmental communication for thorough impact assessments.
Preventive Actions:
Establish continuous change control training for all employees, mandate quality unit reviews for draft changes, and introduce a cross-departmental change impact assessment tool.
Reference FDA 483:
Company Name: Kilitch Healthcare India Limited
Issue Date: 20-Oct-23
Inspection Dates: 12 Oct 2023 to 20 Oct 2023
Investigators: Anastasia M Shields, Justin A Boyd
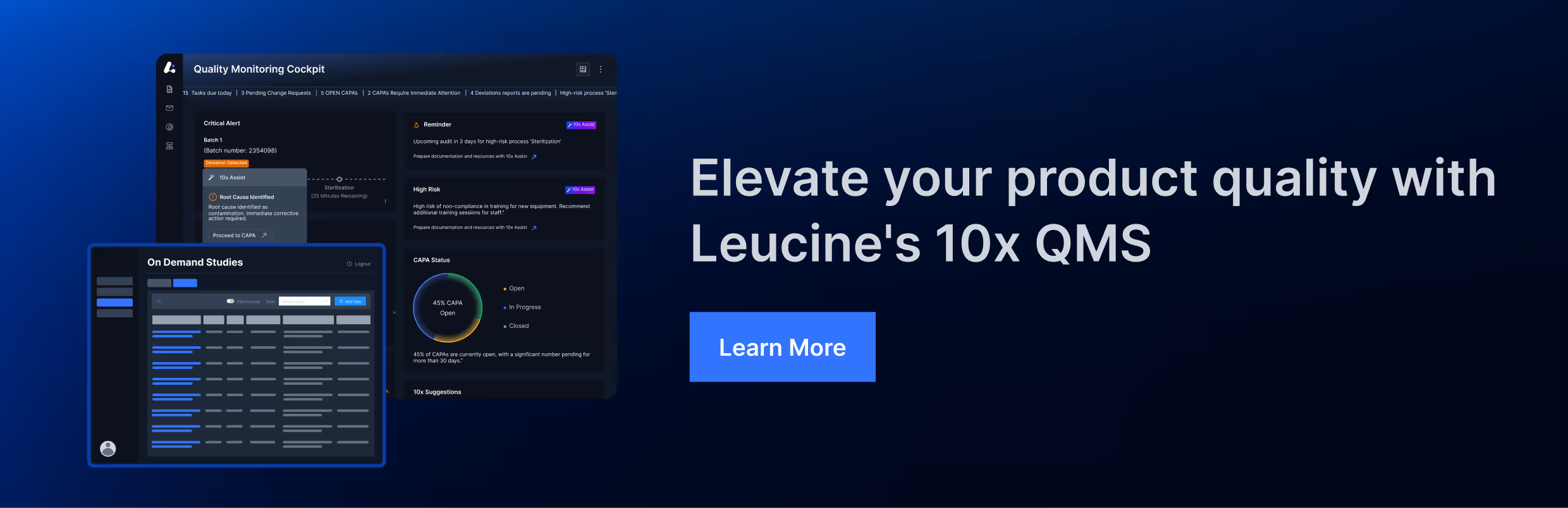
3. Inadequate QCU Review of Procedure Changes
Root Cause:
Non-adherence to SOP KHIL/RB/QA/016/-R07, inadequate training on change documentation, poor inter-departmental communication on formulation changes, and insufficient risk assessments for product quality and patient safety impacts.
Corrective Actions:
Review and reinforce the change control procedure with comprehensive staff training. Implement a robust tracking system for all changes, enhance communication between departments, and review products for potential patient safety risks due to material grade changes.
Preventive Actions:
Develop training on change control, audit records, establish cross-functional review team, and incorporate risk management practices and mitigation strategies.
Reference FDA 483:
Company Name: SCA Pharmaceuticals, LLC
Issue Date: 20-Oct-23
Inspection Dates: 18 Sep 2023 to 20 Oct 2023
Investigators: Jonah S Ufferfilge, John P Mistler
4. Failure to Adhere to Established Process Control Procedures
Root Cause:
Non-adherence to documentation and labeling procedures, lack of real-time printer access, inadequate change control for equipment, and ineffective training causing improper execution of procedures.
Corrective Actions:
Revise documentation procedures, ensure real-time printer access, implement rigorous equipment change control, and retrain staff on procedures and policies.
Preventive Actions:
Establish robust training on procedures and documentation, conduct regular audits, implement real-time tracking, and strengthen change control with detailed, validated guidelines.
Reference FDA 483:
Company Name: Sichuan Deebio Pharmaceutical Co. Ltd.
Issue Date: 08-Sep-23
Inspection Dates: 04 Sep 2023 to 08 Sep 2023
Investigators: Arsen Karapetyan, Anders W Evenson
5. Lack of Proper Drafting, Review, and Approval
Root Cause:
No protocols for initiating change controls for equipment installations, lack of systematic documentation, and inadequate training on change control processes.
Corrective Actions:
Implement a comprehensive change control procedure for equipment and procedural changes, conduct staff training, and assign a coordinator or team for oversight and compliance.
Preventive Actions:
Establish internal audits to assess change control compliance, integrate change control into quality assurance culture, and use automated systems for documentation and management.
Reference FDA 483:
Company Name: Central Admixture Pharmacy Services Inc
Issue Date: 25-Aug-23
Inspection Dates: 10 Jul 2023 to 25 Aug 2023
Investigators: Jolanna A Norton, Rachel C Stanton, Doan N Singh, Xiaohui Shen
6. Inadequate Drafting and Approval of Procedures
Root Cause:
Lack of Quality Unit oversight in reviewing documentation, inadequate change control procedures, and training gaps on document control and procedural adherence.
Corrective Actions:
Revamp document and change control procedures for strict review and approval measures, conduct comprehensive training for all personnel, and retrospectively review past year's changes for proper documentation and approval.
Preventive Actions:
Implement regular audits for compliance with document and change control procedures, establish continuous training for new and existing staff, and enhance Quality Unit oversight to enforce standards.
Reference FDA 483:
Company Name: Novo Nordisk A/S
Issue Date: 04-May-23
Inspection Dates: 27 Apr 2023 to 04 May 2023
Investigators: Prabhu P Raju, Unnee Ranjan, Mikhail Ovanesov, Sergey Akimov

7. Unreviewed and Unapproved Procedure Changes
Root Cause:
No established change control process for pre-implementation documentation and approval, inadequate training on formal change control, and Quality Control Unit lacking oversight or authority to enforce compliance.
Corrective Actions:
Establish formal change control with Quality Control approval, conduct training, and review past uncontrolled changes for quality and safety impacts.
Preventive Actions:
Implement regular audits for change control compliance, enhance Quality Control oversight, and establish a tracking system for timely review and approval of changes.
Reference FDA 483:
Company Name: Cipla Limited
Issue Date: 17-Feb-23
Inspection Dates: 06 Feb 2023 to 17 Feb 2023
Investigators: Saleem A Akhtar, Jose E Melendez
Observations in 2025

.png)
.png)

.png)






















