FDA Observations Complaint Management

1. Inadequate Complaint Handling Procedures
Root Cause:
Lack of comprehensive procedures, insufficient training, inadequate trend analysis, failure to escalate complaints, and unclear third-party call center guidance.
Corrective Action:
Revise SOPs, implement robust training, enhance trend analysis, investigate recurring complaints, and provide guidelines for third-party call centers.
Preventive Action:
Establish continuous improvement, robust training, advanced analytical tools, maintain quality dashboard, and hold regular review meetings with third-party providers.
Reference FDA 483:
Company Name: Sun Pharmaceutical Industries Limited
Issue Date: 15-Dec-23
Inspection Dates: 04 Dec 2023 to 15 Dec 2023
Investigators: Pratik S Upadhyay
2. Inadequate Investigation of Unexplained Discrepancies
Root Cause:
Lack of microbial contamination assessment procedures, insufficient training, and unclear escalation criteria for complaint investigations.
Corrective Action:
Revise procedures for microbial assessment, train staff on new procedures, and introduce Quality Control review for microbial-related complaints.
Preventive Action:
Conduct routine audits, enhance complaint intake for microbial indicators, and periodically review microbial assessment criteria based on standards and guidance.
Reference FDA 483:
Company Name: Kilitch Healthcare India Limited
Issue Date: 20-Oct-23
Inspection Dates: 12 Oct 2023 to 20 Oct 2023
Investigators: Anastasia M Shields, Justin A Boyd
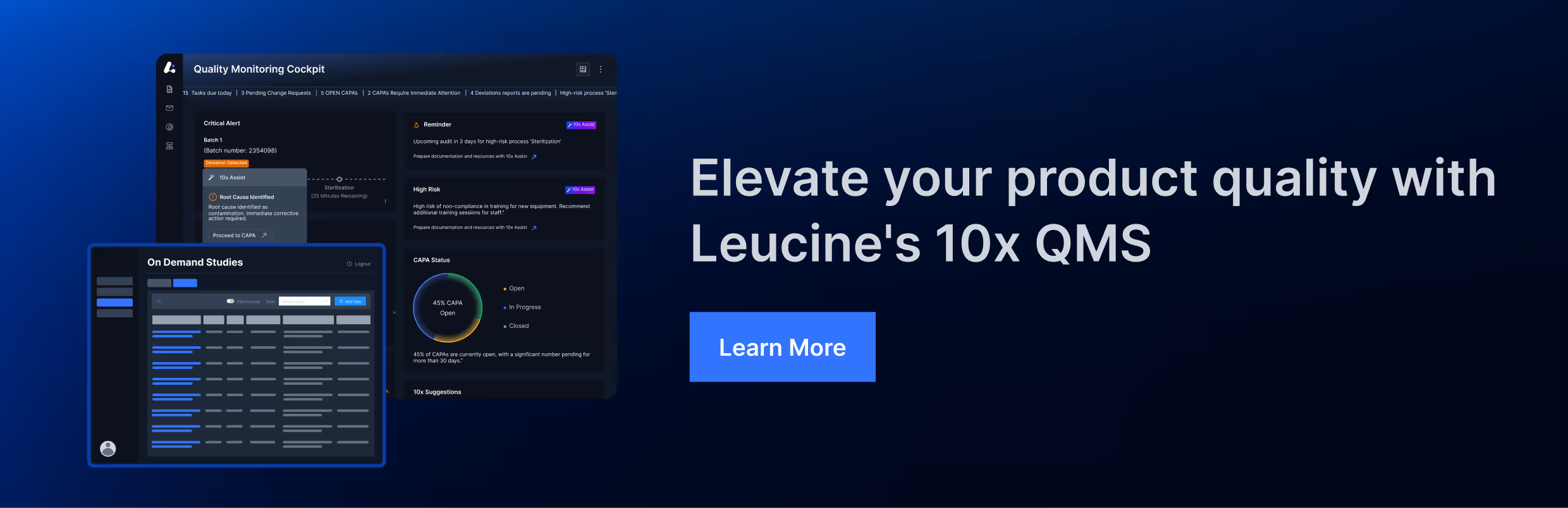
3. Deficient Complaint Procedures Impact Drug Safety Evaluation
Root Cause:
Lack of thorough complaint procedures, inefficient product testing, and insufficient employee training.
Corrective Action:
Revise complaint procedures, implement product testing, and train employees on updated procedures.
Preventive Action:
Periodically review complaint procedures, conduct regular training, and implement a tracking system for complaints and investigations.
Reference FDA 483:
Company Name: SCA Pharmaceuticals, LLC
Issue Date: 20-Oct-23
Inspection Dates: 18 Sep 2023 to 20 Oct 2023
Investigators: Jonah S Ufferfilge, John P Mistler
4. Missing Conclusions and Follow-Up in Discrepancy Records
Root Cause:
Unclear investigation procedures, insufficient training, inadequate documentation, and failure to extend investigations to impacted batches and critical processes.
Corrective Action:
Develop comprehensive SOPs, conduct thorough training, enhance record-keeping, and expand investigation scope to include impacted batches and equipment.
Preventive Action:
Regular training programs, periodic review by senior quality personnel, implement digital tracking, and adopt a risk-based approach for investigations.
Reference FDA 483:
Company Name: Sichuan Deebio Pharmaceutical Co. Ltd.
Issue Date: 08-Sep-23
Inspection Dates: 04 Sep 2023 to 08 Sep 2023
Investigators: Arsen Karapetyan, Anders W Evenson
5. Unreviewed Discrepancies and Batch Specification Issues
Root Cause:
Superficial investigations, lack of trained personnel, inadequate QMS, poor risk evaluation, delayed complaint responses, and insufficient environmental control.
Corrective Action:
Strengthen investigation procedures, review QMS, enhance training, implement escalation processes, expedite complaint handling, and upgrade environmental controls.
Preventive Action:
Ensure CAPA effectiveness, regular staff training, update SOPs, integrate risk management, and promote a culture of transparency in reporting quality issues.
Reference FDA 483:
Company Name: Central Admixture Pharmacy Services Inc
Issue Date: 25-Aug-23
Inspection Dates: 10 Jul 2023 to 25 Aug 2023
Investigators: Jolanna A Norton, Rachel C Stanton, Doan N Singh, Xiaohui Shen

6. Missing Investigation Findings and Follow-Up in Records
Root Cause:
No standardized complaint investigation procedure, insufficient training, inadequate quality oversight, lack of trend analysis tools, and ineffective inter-departmental communication.
Corrective Action:
Revise SOPs for complaint handling, implement targeted training, establish quality oversight mechanisms, and enhance trend analysis technology.
Preventive Action:
Institute continuous training, upgrade IT systems for trend analysis, strengthen inter-departmental communication, and regularly review documentation processes in CAPA system.
Reference FDA 483:
Company Name: Novo Nordisk A/S
Issue Date: 04-May-23
Inspection Dates: 27 Apr 2023 to 04 May 2023
Investigators: Prabhu P Raju, Unnee Ranjan, Mikhail Ovanesov, Sergey Akimov
7. Inadequate Procedures for Managing Drug Complaints
Root Cause:
Undefined complaint handling procedures, lack of training, inadequate quality control oversight, and inefficient complaint tracking system.
Corrective Action:
Revise and document complaint procedures, implement comprehensive training, enhance quality control oversight, and upgrade the complaint tracking system.
Preventive Action:
Establish regular audits, periodically review complaint processes, implement feedback loops, and conduct regular training refreshers for staff.
Reference FDA 483:
Company Name: Cipla Limited
Issue Date: 17-Feb-23
Inspection Dates: 06 Feb 2023 to 17 Feb 2023
Investigators: Saleem A Akhtar, Jose E Melendez

























.png)
