A Complete Guide to Elogbook in Pharmaceutical Manufacturing
Explore how paperless elogbooks and electronic logbook software improve compliance, data integrity, and efficiency in pharma and life sciences operations.


Introduction
In pharmaceutical manufacturing, keeping accurate and compliant records is not just a regulatory requirement — it’s the foundation of product quality and patient safety. Yet, many facilities still use paper-based logbooks to track equipment usage, cleaning, calibration and other routine tasks. These paper records are time consuming, error prone and increasingly incompatible with modern regulatory expectations.
That’s why the move to elogbooks — or electronic logbooks — is happening across the life sciences industry. These digital systems help manufacturers simplify documentation, improve operational efficiency and reduce errors through automation, ensuring data accuracy. Automated systems like AI driven digital logbooks reduce manual documentation errors and ensure accurate data collection, ultimately compliance and data integrity. And data security is key to safeguarding sensitive information through robust features like encryption and access controls.
In this guide we’ll explore how pharmaceutical companies can move from paper to a paperless elogbook system, the benefits of electronic logbook software and how Leucine’s Production Logbook supports audit ready, connected operations within a Pharma MES environment.
What is an Elogbook?
An elogbook is a digital solution to replace traditional paper-based logbooks, a more efficient and effective way to record and manage data. In short it’s a software application that captures, stores and manages data electronically. This move from paper to digital is particularly beneficial in industries like pharmaceutical manufacturing and life sciences where data integrity, accuracy and security is paramount. Unlike paper-based logbooks, elogbooks provide real-time data capture, automated checks and centralised oversight, so all records are accurate, complete and easily accessible.
Definition and Purpose of Elogbooks in Pharmaceutical Manufacturing
In pharmaceutical manufacturing elogbooks play a critical role in recording and managing data related to production processes, equipment usage and quality control. The purpose of these digital logbooks is to provide a secure, accurate and efficient way to capture and store data, so compliance with strict regulatory requirements and industry standards. By using elogbooks pharmaceutical companies can maintain data integrity, reduce errors and improve operational efficiency. This digital approach simplifies documentation and supports real-time monitoring and traceability which is essential for high quality and safety.
From Paper to Paperless: The Evolution of Logbooks
Traditionally pharmaceutical teams have used paper logbooks to record operational data — equipment cleaning, preventive maintenance or shift handovers. While simple, these manual systems are error prone and compliance challenged. The move to e logbook systems has brought many efficiencies through log centralization and digital management, data integrity and compliance and operational workflow.
Key limitations of paper logbooks are:
- Incomplete or illegible entries
- No real time visibility
- Difficult to ensure ALCOA+
- Time consuming reviews and approvals
- Inefficiency during audits and inspections
Similarly, the life sciences sector is also transitioning to elogbooks to enhance data management and ensure regulatory compliance.
The shift to digital logbook software addresses these challenges by enabling electronic data capture, automated checks and centralised oversight — all in line with 21 CFR Part 11 regulations.
Paper Based Logbook Challenges
Paper based logbooks have been around for centuries but come with several inherent limitations and drawbacks especially in fast paced manufacturing environments. These traditional methods are seen as outdated and inefficient in today’s digital age.
1. Limitations and Drawbacks of Traditional Paper Based Logbooks
Paper based logbooks are fraught with challenges that can hinder data management. They are error prone, inconsistent and data loss can compromise the accuracy and reliability of the recorded information. Managing these logbooks is time consuming and labour intensive, requires manual effort for data entry and review. Paper based logbooks lack robust security features making them vulnerable to unauthorized access and tampering. Scaling these systems to accommodate growing data volumes can be difficult and retrieving specific data points can be a cumbersome process. These limitations highlight the need for a more modern digital approach to data management.
2. Errors and Inconsistencies in Data Entry and Documentation
One of the biggest drawbacks of paper based logbooks is human error. Inaccurate or inconsistent data entry can lead to compliance risks and regulatory issues, undermine the whole documentation process. These errors can also cause operational inefficiencies as incorrect data can lead to misguided decisions and actions. Electronic logbooks offer a secure and accurate way to capture and store data, reduces the risk of errors and inconsistencies. By automating data entry and validation checks electronic logbooks ensure all records are accurate and reliable, overall operational efficiency and compliance improves.In summary electronic logbooks offer a better alternative to paper based logbooks, data integrity, reduced errors and improved operational efficiency. By moving to digital logbooks pharmaceutical manufacturers can meet regulatory requirements, simplify their operations and manage data better.
Why the Pharma Industry is Moving to Elogbooks
The adoption of elogbooks in pharmaceutical manufacturing is not a trend — it’s a necessity towards digitalisation. Regulatory bodies now expect systems to maintain data integrity, ensure traceability and minimise human error.
The life sciences sector is also recognizing the necessity of elogbooks to maintain data integrity and ensure compliance.
A digital operations logbook provides business and compliance benefits:
- Real Time Data Capture: Operators log activities directly on tablets or workstations on the shop floor.
- Compliance by Design: Time stamped entries, restricted user roles and automatic audit trails support ALCOA+ and Part 11.
- Streamlined Review Cycles: QA teams can review entries remotely and flag deviations in real time.
- Centralised Access: Managers have full visibility of daily operations across equipment, areas and teams.
- Reduced Paper Dependency: Save storage space, reduce carbon footprint and eliminate printing and scanning hassles.
By going paperless elogbook pharma companies reduce compliance risks and lay the foundation for integrated digital ecosystems like Pharma MES platforms.
Benefits of Electronic Logbook Software

An effective electronic logbook software goes beyond digital record keeping — it changes operational culture. In addition to pharmaceutical manufacturing, electronic logbook software offers significant benefits to the life sciences sector by enhancing data management and ensuring regulatory compliance. Here’s how Leucine’s Production Logbook delivers value to your teams:
1. Compliance and Data Integrity
Every action is automatically time stamped and linked to the authorised user. The system prevents backdated entries, ensures SOP adherence and maintains an immutable audit trail.
2. Real Time Visibility
Supervisors and QA can see log status in real time — whether logs are complete, pending or overdue. This allows faster intervention and fewer missed tasks.
3. Reduced Review Burden
Digitised workflows mean logs don’t pile up waiting for review. Reviewers can access records instantly, leave comments and approve entries with electronic signatures.
4. Operator Friendly Design
With mobile first forms, guided workflows and simple interfaces even floor-level operators can adapt to the system quickly, reducing training time and errors.
5. Integration with Pharma MES
Leucine’s Production Logbook integrates with existing systems, including various operational platforms like ERP and MES, as well as other modules like batch records, deviation management and maintenance planning — a single operational framework.
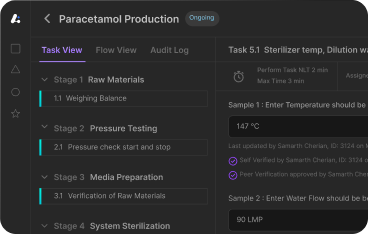
How Production Logbook by Leucine Changes Daily Operations
Leucine’s Production Logbook is a purpose built digital logbook software for pharmaceutical manufacturing. It automates and digitises daily operations — from cleaning records and line clearance to shift handovers, shift turnover and environmental checks — so teams can stay compliant, efficient and audit ready. Leucine’s Production Logbook is also widely used in the life sciences sector to automate and digitize daily operations, ensuring compliance and efficiency.
Here’s what sets it apart:
- Zero Downtime Deployment: Deploy in weeks, not months.
- Customisable Templates: Tailor log formats to match existing SOPs without disrupting workflows.
- Smart Alerts & Escalations: Automatically notify teams when log entries are overdue or out of compliance.
- Audit Ready Anytime: Retrieve any log, any time from a central dashboard with full traceability.
- Works Across Devices: Accessible via tablets, desktops and mobile — optimised for shop floor use.
- Multiple Logbooks: Create multiple logbooks to differentiate between various entries and simplify the search process, for plant managers to have better oversight and efficiency.
With Production Logbook you get the flexibility of a paperless elogbook without compromise on compliance or control.
Conclusion
In today’s fast paced pharmaceutical world electronic logbook software isn’t a nice to have — it’s a must. From regulatory compliance to operational excellence the benefits of a paperless elogbook are clear.
In the life sciences sector, electronic logbook software is essential for maintaining data integrity and ensuring regulatory compliance.
Leucine’s Production Logbook helps you eliminate paperwork, strengthen your data integrity and accelerate your digital transformation — all while staying audit ready, every day.
Ready to Go Digital?
🎯 Get a Live Demo of Production Logbook
See how it works for your site with real time workflows and templates.
📥 Download the Elogbook Implementation Checklist
Start planning your transition from paper to digital with confidence.
👥 Talk to an Expert
Speak with our compliance and operations specialists about your site’s unique needs.

View and learn more about Cleaning Validation with our comprehensive list of resources






























